Lab scale producing of drug substances and drug items, manufacture of medical materials for scientific reports, scaling as many as commercial batch dimensions, business merchandise.
This segment is relevant only for orphan medicines. Expected info on aspects and technique are current in “European Commission guideline on elements of the applying of Post 8 of Regulation (EC) No 141/2000: Assessment of similarity and/or clinical superiority of orphan medicinal products when assessing marketing authorization applications and variations.”
Examining done batch generation and laboratory Command records of critical course of action ways ahead of release with the API for distribution
To avoid audit results and citations — plus the probable for being forced to put into action a recall due to very poor documentation practices — very good recordkeeping is crucial.
ASMFs holders must submit their file for the MHRA. It can be your responsibility to ensure that you submit the ASMF either before you decide to submit your application or concurrently. Your software will not be legitimate without it.
Within the globe Group, elements may fluctuate as to their lawful classification being an API. When a material is assessed being an API inside the location or place during which it can be created or used in a drug solution, it ought to be made according to this steerage.
Because of this, frequently rising digital developments throughout the market grew to become the driving force for numerous pharma firms that comply with them to boost their capabilities.
He has rich information and provides worthwhile insights and information via his more info content articles and information on Pharmaguddu.com. For more inquiries or collaborations, please don’t wait to achieve out by using website e mail at Get hold of@pharmaguddu.com.
The corporate must designate and doc the rationale for The purpose at which production of the API starts. For synthetic processes, this is known as the point at which API starting off materials are entered into the method.
Produce acceptable requirements/controls for drug substance and drug product to be certain protection, efficacy and good quality.
Sign up with the professional e mail to avail Specific improvements supplied in opposition to purchase orders, seamless multi-channel payments, and extended support for agreements.
Ans: Agent Sampling will be the sampling from the different layers & a composite sample is ready eg. composite samples in the container are sampled.
To aid Within this field, We've curated a list of one hundred+ High-quality Assurance Job interview Thoughts suitable for both entry-level and professional candidates. We belief that these concerns, specializing in Top quality Assurance/IPQA, will information you toward knowing your profession aspirations while in the pharmaceutical sector.
The implications of not adhering to GDP in many cases are extreme and the businesses can get into major troubles for not subsequent the right insurance policies/suggestions.
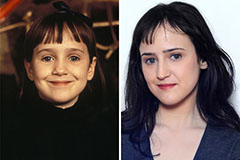 Mara Wilson Then & Now!
Mara Wilson Then & Now! Tatyana Ali Then & Now!
Tatyana Ali Then & Now! Brandy Then & Now!
Brandy Then & Now! Danielle Fishel Then & Now!
Danielle Fishel Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!